The cell membrane separates the contents of the cell with its environment, but in order to function well it is necessary that a certain permeability of chemical substances exists. But the degree of permeability is different for different substances: we therefore speak about semi-permeability. Potassium, sodium, and chloride are three ion species for which the membrane is permeable. This is due to the presence of pores and specific channels in the membrane, called ionic channels, which operate without energy consumption. In addition, there are pumps and ion-exchangers in the lipid bilayer that do consume energy in order to regulate compositions within and outside the cell. They are necessary because otherwise ion compositions eventually would not remain intact.
The membrane potential is the result of the semi-permeability of the cell membrane, and two processes in the cell membrane play a role:
- diffusion of an ion through the membrane as a result of differences in concentration inside and outside the cell;
- movement of ions in an electric field.
The permeability of the potassium ion is a key player because this is for a cell 'at rest' is the most permeable ion species and the so-called \(\mathrm{K}^{+}\) channel contributes much to the establishment of the membrane potential. But we will explain the theory of an arbitrary ion species, even though you may think here of \(\mathrm{K}^{+}\) and a\(\mathrm{K}^{+}\) channel. In any case, we start with the special situation in which there is only one ion \(\mathrm{X}^{+}\), having a homogeneous concentration greater inside than outside the cell, and only one type of channel for this ion playing a role. Let us take in this case a close look at the two processes.
The concentration gradient exerts a diffusion force on the ions, that makes them move from high to low concentration regions. However, since ions having a charge, the motion of the ions also establishes an electrostatic force that is opposite in direction to that of the diffusion force. As more ions streams from regions of high to low concentration, the electrostatic force increases. At a certain moment, these two forces are in balance. The equilibrium potential for the particular ion is then called the Nernst potential of the ion. Nernst's theorem, also called the Nernst equation, relates the equilibrium concentrations and the Nernst potential.
The equilibrium potential of an ion is given by \[E_\mathrm{ion}= \frac{RT}{zF}\cdot \ln\left(\frac{C_{\mathrm{e}}}{C_{\mathrm{i}}}\right) = -\frac{RT}{zF}\cdot \ln\left(\frac{C_{\mathrm{i}}}{C_{\mathrm{e}}}\right)\] This potential represents the resting membrane potential in a model of the cell with a single ion species and a single type of ion channel, and the value depends on the absolute temperature \(T\), the valence \(z\) (the formal charge of an ion measured in number of charge units), and the ion concentrations inside (\(C_{\mathrm{i}}\)) and outside (\(C_{\mathrm{e}}\)) the cell near the membrane. \(R\) is the gas constant and \(F\) Faraday's constant (equal to the load of one mole of monovalent ions).
Diffusion
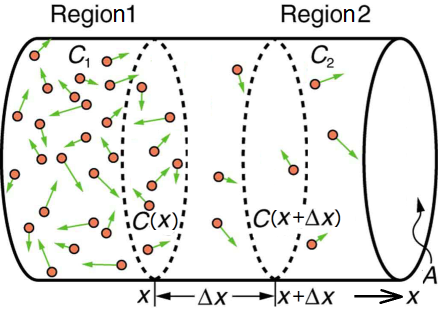
Physical chemistry teaches us that there is a movement of ions from areas of high to low concentration, and that the flux \(\Phi\), that is, the net movement of ions per unit time per unit surface region in the direction of the decreasing concentration, is given by Fick's law: \[\Phi= -D\cdot \frac{\dd C}{\dd x}\] where \(D\) is the diffusion coefficient and \(\dfrac{\dd C}{\dd x}\) is the component of the gradient of the concentration that points in the direction in which the transport (local) is happening (in the drawing below the positive \(x\) direction). The minus sign takes into account that the direction of transport is from areas with a high concentration to areas of low concentration. The figure below illustrates this process and the formula \[\Phi(x)\cdot \Delta x \approx -D\cdot \bigl(C(x+\Delta x)-C(x)\bigr)\] symbolizes that the number of particles that flows into the volume between \(x\) and \(x+\Delta x\) per unit of time is proportional to the concentration difference between the locations \(x\) and \(x+\Delta x\) at that time.
Einstein and Nernst have derived the following formula for the diffusion constant of a charged particle: \[D=\frac{RT}{F}\cdot u\] where \(R\) is the gas constant, \(T\) the absolute temperature (in Kelvin), \(F\) the Faraday constant (the charge of one mole of monovalent ions) and \(u\) the 'mobility' of the ion. When one mole of ions with valence \(z\) (the number of unit charges per ion) diffuses we can interpret this as an electric flow corresponding with the current \[I_D= zF\cdot \Phi = - zRT\cdot u\cdot \frac{\dd C}{\dd x} \]
Movement of ions in an electric field
The diffusion of ions from a region of high concentration to a region of low concentration, which are separated from each other by a semipermeable membrane, results in a charge difference near the membrane. Suppose that the ion is positively charged, has a high concentration within the cell, and has a low concentration outside the cell, then diffusion causes that negative charge remains on the inside of the membrane and that outside the cell near the membrane positive charge accumulates. The membrane is in this way a capacitor and as more ions diffuse this charge difference increases. But this also means that there is an increasing potential difference in the vicinity of the membrane. The diffusion of the ions will therefore become increasingly more difficult because of opposing electrical forces. The flux \(\Phi_E\) as a result of the electric field \(E\) that corresponds with the potential difference \(V\) is given by the following formula: \[\Phi_E=-z\cdot u\cdot C\cdot \frac{\dd V}{\dd x}\] where \(z\) again is the valence of the ion and \(\dfrac{\dd V}{\dd x}\) is the potential difference per distance. When one mole of particles having valence \(z\) moves in the electric field, we can interpret this as an electric flow with a current \[I_E=zF\cdot\Phi_E = -z^2F\cdot u\cdot C\cdot \frac{\dd V}{\dd x}\]
The Nernst equation
There establishes little by little an equilibrium in which the diffusion and the movement of the ions in an electric field have the same size, but are oppositely directed; we say that the cell is 'at rest', but there actually is movement of ions through the membrane, but the inflow and outflow are equal to each other. In other words, in equilibrium, the sum of the electric current because of diffusion and movement of ions in an electric field, \(I_D+I_E\), is equal to zero. So \[I_D+I_E = -z\cdot u\cdot \!\!\left(RT\cdot \frac{\dd C}{\dd x}+zFC\cdot \frac{\dd V}{\dd x}\right)=0\] In equilibrium, the term between brackets must be equal to zero, and this gives: \[-\frac{RT}{zF}\cdot \frac{1}{C}\cdot \frac{\dd C}{\dd x}= \frac{\dd V}{\dd x}\] This implies: \[-\frac{RT}{zF}\cdot \frac{\dd\ln(C)}{\dd x}= \frac{\dd V}{\dd x}\] Therefore, when we integrate over the lipid bilayer and apply the rule \(\ln(x)-\ln(y)=\ln(x/y)\), then we get \[-\frac{RT}{zF}\ln\left(\frac{C_\mathrm{e}}{C_\mathrm{i}}\right)=V_\mathrm{e}-V_\mathrm{i}\] So: \[V_\mathrm{i}-V_\mathrm{e}= \frac{RT}{zF}\ln\left(\frac{C_\mathrm{e}}{C_\mathrm{i}}\right)\] On the left-hand side we have a potential difference between the interior and exterior of the membrane for a cell at rest, but in electrophysiology it is common practice to set the reference potential on the outside of the cell near the membrane equal to zero. For a positively charged ion with a higher concentration on the inside of the cell at rest, the potential will then have on the inside of the cell near the membrane a negative value, which we call the Nernst potential or equilibrium potential of the ion and denote by \(E_\mathrm{ion}\). The equation found is called the Nernst equation.
In the above argument we have in fact assumed that ionic currents are so small that the concentrations inside and outside the cell do not alter. We also did not include osmotic pressure, i.e., we have assumed that this is inside and outside the cell the same. In animal cells, these assumptions are valid.
Let us make an estimate for potassium ions in a cell with Nernst potential \(E\) of \(-60\,\mathrm{mV}\). For the specific capacity \(C\) membrane (i.e. capacitance per unit region) we can take a reasonable estimate of\(1\,\mathrm{\mu F/cm}^2\). This means that the charge \(Q\) on the membrane per unit region is given by \[Q=C\cdot E= (10^{-6}\,\mathrm{F/cm}^2)\cdot (60\cdot 10^{-3}\,\mathrm{V})=6\cdot 10^{-8}\,\mathrm{C/cm}^2\] A charge of 1 farad or about 96500 C, corresponds to 1 mole of the monovalent ion. We can calculate in this way the number of ions to be moved calculate per square centimetre; this number is in moles approximately equal to \[\frac{6\cdot 10^{-8}\,\mathrm{C/cm}^2}{96500\,\mathrm{C/mol}}\approx 6\cdot 10^{-13}\,\mathrm{mol/cm}^2\] When we multiply this number by the Avogadro's constant, this leads to \[(6\cdot 10^{-13}\,\mathrm{mol/cm}^2)\cdot (6\cdot 10^{23}\,\mathrm{mol}^{-1})=3.6\cdot 10^{11}\, \mathrm{cm}^{-2}\] This means that 3600 ions per \(\mu \mathrm{m}^2\) are transported. Suppose that the cell is spherical with a diameter \(d=10\,\mu\mathrm{m}\). Then we have a surface area of \(\pi\cdot d^2\) and the total number of transported ions is equal to \(3600\cdot \pi\cdot 100\approx 1.13\cdot 10^6\). The volume of the cell is equal to \(\frac{1}{6}\pi\cdot d^3\) and in this case approximately equal to \(524\,\mu\mathrm{m}^3\). Suppose an ion concentration of 100 mM (i.e. 100 mol/m3), then this corresponds to a number of ions equal to \[(100\,\mathrm{mol}/\mathrm{m}^3)\cdot (524\cdot 10^{-18}\,\mathrm{m}^3) \cdot (6\cdot 10^{23} \,\mathrm{mol}^{-1})=3.14\cdot 10^{10}\] This means that in a spherical cell with a diameter of \(10\mu\mathrm{m}\) about \(1.13\cdot 10^6\) ions must be transported, where we have in total \(3.14\cdot 10^{10}\) ions, in order to achieve an electric potential of \(-60\,\mathrm{mV}\). So, the change in the ion concentration within the cell by the ion transport is only 0.004%. We can therefore neglect it in calculations for a short time period.
Let us also substitute concrete numbers: \(F\approx 96485\;\mathrm{C}\,\mathrm{mol}^{-1}\), gas constant \(R\approx 8.315\,\mathrm{J}\,\mathrm{K}\,\mathrm{mol}^{-1}\), temperature \(29.25\,{}^{\circ}\mathrm{C}\), i.e. \(T\approx 302.4\;K\). Then we have \(E_\mathrm{ion} \approx -\frac{0.0261}{z}\ln\left(\frac{C_\mathrm{i}}{C_\mathrm{e}}\right)\) (in volts). It is more convenient not to work with the natural logarithm, but with the base 10 logarithm, and to use the unit mV for the electric potential difference. Then we get the following formula for the equilibrium potential of an ion: \[E_\mathrm{ion}\approx -\frac{60}{z}\cdot \log_{10}\left(\frac{C_\mathrm{i}}{C_\mathrm{e}}\right)\quad\text{(in mV)}\] Please note that this is the equilibrium potential at the given temperature \(29.25\,{}^{\circ}\mathrm{C}\): at body temperature (\(37\,{}^{\circ}\mathrm{C}\) ) the coefficient in the formula for the Nernst potential is equal to \(61.5\,\mathrm{mV}\) instead of \(60\,\mathrm{mV}\), and at a temperature of \(20\,{}^{\circ}\mathrm{C}\) the coefficient in the formula for the equilibrium potential is equal to \(58.2\,\mathrm{mV}\).
For convenience, we will use in the rest of this section only the base 10 logarithm and simply write \(\log\) instead of \(\log_{10}\). We will also employ, unless otherwise stated, a temperature of \(29.25\,{}^{\circ}\mathrm{C}\) so that the equilibrium potential of an ion can be calculated (in mV) through the formula \[\begin{aligned}E_\mathrm{ion}&=-\frac{60}{z}\cdot \log_{10}\left(\frac{C_\mathrm{i}}{C_\mathrm{e}}\right)\\ \\ &= \frac{60}{z}\cdot \log_{10}\left(\frac{C_\mathrm{e}}{C_\mathrm{i}}\right)\quad\text{(in mV)}\end{aligned}\] In this case of a monovalent ion the equilibrium potential decreases by 60 mV for each factor of 10 difference in concentration inside and outside the cell. The table below shows the equilibrium potential for a number of biologically relevant ions based on the previously in the introduction given concentrations for a cell at rest. Check these values and pay attention to the valence of the ion.
\[
\begin{array}{l|rrrr}
\mathrm{ion\;} X & [X]_\mathrm{e} & [X]_\mathrm{i} & [X]_\mathrm{e}\mathrm{\;:\;}[X]_\mathrm{i} & E_\mathrm{ion}\;(\mathrm{mV})\\ \hline
\mathrm{K}^{+} & 5 & 100 & 1\mathrm{\;:\;}20 & -78 \\
\mathrm{Na}^{+} & 150 & 15 & 10\mathrm{\;:\;}\phantom{2}1 & +60\\
\mathrm{Ca}^{2+} & 2 & 0.0002 & 10000\mathrm{\;:\;}\phantom{2}1 & +120 \\
\mathrm{Cl}^{-} & 150 & 13 & 11.5\mathrm{\;:\;}\phantom{2}1 & -64\\
\end{array}\]
 The Nernst potential for an ion species
The Nernst potential for an ion species



